Anti-counterfeiting or ACF technology is becoming in greater demand in all industry sectors. It’s coming at a time when major product brands are being attacked by large shady, but technologically savvy organizations that are out to make big and fast money.
Paul Karazuba, Senior Director, Product Marketing, and Scott Best, Technical Director, for Rambus’ Cryptography Products Division, each wrote an article in major medical equipment publications to call attention to this problem and prescribed the best cures for ACF.
Karazuba’s article appeared in MD&DI, while Best’s article is in Medical Design Technology Magazine (Part 1| Part 2). See links to articles at the bottom of this blog.
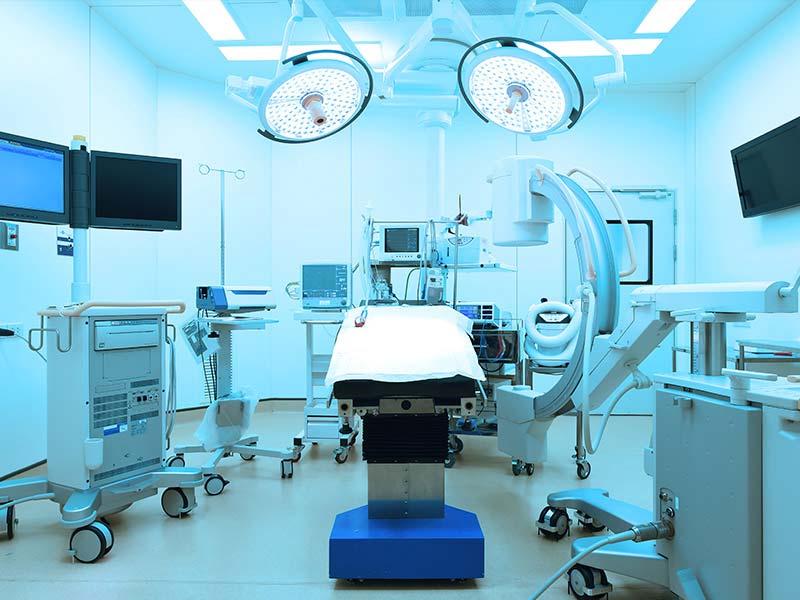
Medical equipment is one of the hardest hit sectors, according to these Rambus authors. Karazuba points out that “Counterfeit medical devices and equipment enter the legitimate healthcare supply chain in many instances because buyers are looking at their bottom line and seeking the best price. This is an open invitation to counterfeiters to hit those lower price points with their fake goods.”
He adds that major concerns focus on patient safety relating to counterfeit medical equipment. Those non-FDA qualified devices and equipment pose the greatest health risks to medical patients. Here, the spectrum is extensive going from inaccurate diagnosis on to injury and even death. There’s also the associated potential OEM liability and brand issues that come into the picture.
According to Karazuba and Best, the best route for medical equipment OEMs to take against counterfeiting is to adopt a hardware prover chip solution together with verifier software, a secure manufacturing flow, and a challenge/response protocol between verifier and prover—all in one platform for the medical equipment OEM.
The authors call attention to the problem of differential power analysis or DPA, which gives the adversary carte blanche for counterfeiting operations. However, the Rambus ACF solution generates the response to a challenge in such a way that is immune from DPA attacks.
“At the secure manufacturing flow stage, the prover chip undergoes specific and controlled steps during the manufacturing process before it goes to the medical equipment OEM for installation,” Best explains. He adds that this secure manufacturing flow is necessary to prevent theft of an authentic prover chip. “This is the most cost-effective way for a counterfeiter to deliver compatible to the market,” Best says.
To learn more about Rambus ACF solutions, click here.
Leave a Reply